The 2013- 2014 flu season is here for the Northern Hemisphere. Los Angeles has reported it’s first flu case early September (Click here). New York city residents are surprised that flu season has already arrived and reaching people before their scheduled flu vaccination date. The first Los Angeles case is an H1N1 which is in this year’s flu vaccine combination to protect those vaccinated this season. The New York city strain has yet to be reported. Fortunately, the flu vaccine of 2013 – 2014 has either three flu strains (trivalent) or the newly introduced four flu strains (quadrivalent) described more in detail below. If you live in the Northern Hemisphere, you might want to begin flu vaccination plans much earlier this flu season, since the flu has already arrived atleast in two major USA cities and it is only early September. However, in Australia and countries of the Southern Hemisphere, the peak of the 2012 – 2013 flu season is expected to have just ended in August 2013, since the seasons are opposite.
This is what the flu virus looks like
Why should you take the flu vaccine?
The reason one has to take the vaccine every year is because the vaccine only provides immunity for about a year. Unlike the natural infection which gives you lifetime immunity, a flu vaccine does not. Even if in certain years the vaccine flu strain composition is exactly the same as in the previous year. The individual is advised to take the flu vaccine annually. The answer is explained in detail in this previous article.
However, do take your doctor’s advice before vaccination. Certain flu vaccines are not recommended for all ages. For example, CDC recommends that one brand of inactivated flu vaccine called Affluria, should not be given to children 8 years of age or younger. A related vaccine was related with fever and fever – related seizures in young children in Australia. More about side effects below. Young children who get inactivated flu vaccine and pneumococcal vaccine (PCV13) at the same time may be at increased risk for seizures caused by fever. Ask your doctor for more information. Tell your doctor if a child who is getting flu vaccine has ever had a seizure.addition,
Egg and/or latex allergy?
What if you have an egg and/or latex allergy? Consult your doctor. The answer is in this previous article. However, there is a major update for individuals with egg allergy waiting to be vaccinated – Flublok. In 2013, FDA has approved Flublok, for 18-49 year old, which is not developed in eggs but in insects. Hence, Flublok may be suitable for adults (but not children, teens and seniors) with egg and/or latex allergy. Traditionally, the flu vaccine has been developed in eggs. To learn more you may read a previous article, “Tracking the history of the development of the flu vaccine”, by clicking here. The ability to develop the flu vaccine in insects instead of eggs is heralded as a boon to egg allergic individuals. However, since it has been newly introduced in 2013, it remains to be clinically tested in individuals other than adults and hence, is not yet FDA approved in children, teens and seniors, since their immune system is different than a typical healthy adult.
Please, discuss with your doctor. It is highly recommended to take the flu vaccine to avoid hospitalization and other secondary complications from a flu infection. It is highly contagious. Certain age groups are more susceptible than others. The Centers of Disease Control of USA reports a total of 12,343 hospitalizations that occurred from October 1, 2012 through April 30, 2013, which translates to a cumulative rate of 44.3 influenza-associated hospitalizations per 100,000 people in the United States. The total number of influenza-associated pediatric deaths reported to CDC for 2012-2013 was 146 in USA. While the vast majority of the tested virus samples (>99%) showed susceptibility to the antiviral drugs oseltamivir and zanamivir, some varieties showed resistance. Watch an animated video of how the flu virus enters, and multiplies inside the human body.
Why do I feel fatigued after my flu vaccine?
The vaccine composition is changed every year. The WHO meets twice a year to discuss the varieties of flu strains causing flu infections and hopes to include the most “popular” strains in the flu vaccine composition (see below for for the 2013-2014 composition). However, the vaccine can accomodate a maximum of three to four flu strains. There might be a new flu strain that emerges later in the year. There might be a fifth or sixth flu strain also causing infections. The flu vaccine assists an individual in easing the “suffering from symptom” period. Therefore, a person who has the vaccine can still get the flu from a flu strain not included in the vaccine but the symptoms will be weaker. This is because the flu strains differ from each other very slightly and most of their characteristic proteins are included in the vaccine, hence “teaching” the body’s immune system to be ready to fight the flu infection.
There can be a slight fever and other side effects (see below) after the flu vaccine. The symptoms would start right away and last 2 to 3 days. This might happen if the flu vaccine composition includes one or more flu strains that were not included in the previous year’s vaccine composition. If the individual has never been exposed to 1 or more of the flu strains in the vaccine, the immune system might react with symptoms like the flu. It is the immune system getting ready. The individual does not get flu from the inactivated flu vaccine and the live flu vaccine is too weakened to cause the flu. You may also read “Why am I feeling fatigued after taking the flu vaccine?”
The New Quadrivalent Flu Vaccine: Until recently, the vaccine compositions have only had three different kinds (trivalent) of circulating flu varieties. This year, the vaccine choices include those with four different kinds (quadrivalent) of circulating flu varieties, approved by the WHO (see quote below). Do be aware of your vaccine choices in 2013 to 2014 – trivalent or quadrivalent and discuss with your care provider. The FDA approved companies in 2013 manufacturing the new quadrivalent flu vaccine are:
1) GlaxoSmithKline – Fluarix
2) AstraZeneca – MedImmune
3) Sanofi Aventis – Fluzone approved in June 2013 for children 6 months or older, adolescents and adults.
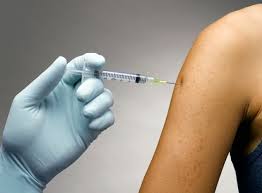
One method of delivery of Flu Vaccine

One method of delivery of flu vaccine
The 2013-2014 Influenza/Flu virus compostion
The compostion of the 2013 – 2014 trivalent and quadrivalent flu vaccine for the Northern Hemisphere:
The World Health Organization has met in Geneva Switzerland in February 2013 to decide upon the composition of the Influenza or flu vaccine composition of the upcoming 2013-2014 flu season. Read more.
Influenza/flu vaccine Adverse Effects
The CDC site has the following safety information. In addition to including three to four different flu varieties in the vaccine composition (trivalent or quadrivalent), the flu vaccine may be either inactivated or live. If inactivated, it can never cause the flu because it does not contain a live virus. However, inactivated flu vaccine could be associated with Guillain-Barré Syndrome (GBS), no more than 1 or 2 cases per million people. If the flu vaccine is live, it is made from a weakened virus and does not cause flu. Both may cause allergic reactions in less than one in million doses. Quote
Influenza (inactivated) vaccine side-effects
What are the risks from inactivated influenza vaccine?
With a vaccine, like any medicine, there is a chance of side effects. These are usually mild and go away on their own.
Serious side effects are also possible, but are very rare. Inactivated flu vaccine does not contain live flu virus, so getting flu from this vaccine is not possible.
Brief fainting spells and related symptoms (such as jerking movements) can happen after any medical procedure, including vaccination. Sitting or lying down for about 15 minutes after a vaccination can help prevent fainting and injuries caused by falls. Tell your doctor if you feel dizzy or light-headed, or have vision changes or ringing in the ears.
Mild Problems
soreness, redness, or swelling where the shot was given
hoarseness; sore, red or itchy eyes; cough
fever
aches
headache
itching
fatigue
If these problems occur, they usually begin soon after the shot and last 1 or 2 days.
Moderate Problems
Young children who get inactivated flu vaccine and pneumococcal vaccine (PCV13) at the same time may be at increased risk for seizures caused by fever. Ask your doctor for more information. Tell your doctor if a child who is getting flu vaccine has ever had a seizure.
Severe Problems
A severe allergic reaction could occur after any vaccine (estimated less than 1 in a million doses).
There is a small possibility that inactivated flu vaccine could be associated with Guillain-Barré Syndrome (GBS), no more than 1 or 2 cases per million people vaccinated. This is much lower than the risk of severe complications from flu, which can be prevented by flu vaccine.
The safety of vaccines is always being monitored. For more information, visit: Vaccine Safety Monitoring and Vaccine Safety Activities.
One brand of inactivated flu vaccine, called Afluria, should not be given to children 8 years of age or younger, except in special circumstances. A related vaccine was associated with fevers and fever-related seizures in young children in Australia. Your doctor can give you more information.
This information was taken directly from the Inactivated Influenza VIS
(This information taken from Inactivated Influenza VIS dated 7/26/2013. If the actual VIS is more recent than this date, the information on this page needs to be updated.)
Influenza (live) vaccine side-effects
What are the risks from LAIV?
With a vaccine, like any medicine, there is a chance of side effects. These are usually mild and go away on their own.
Serious side effects are also possible, but are very rare. LAIV is made from weakened virus and does not cause flu.
Mild Problems
Some children and adolescents 2-17 years of age have reported:
runny nose, nasal congestion or cough
fever
headache and muscle aches
wheezing
abdominal pain or occasional vomiting or diarrhea
Some adults 18-49 years of age have reported:
runny nose or nasal congestion
sore throat
cough, chills, tiredness/weakness
headache
Severe Problems
A severe allergic reaction could occur after any vaccine (estimated less than 1 in a million doses).
The safety of vaccines is always being monitored. For more information, visit: Vaccine Safety Monitoring and Vaccine Safety Activities.
This information was taken directly from the LAIV VIS
(This information taken from Live Influenza VIS dated 7/2/12. If the actual VIS is more recent than this date, the information on this page needs to be updated.)

The WHO headquarters in Geneva, Switzerland

A meeting in action at the WHO headquarters in Geneva, Switzerland
World Health Organization (WHO) headquartered in Geneva, Switzerland recommends the flu vaccine composition each year for the Northern Hemisphere and the Southern Hemisphere.
Related Article:
Review by Anne Sealey: A Cruel Wind: Pandemic Flu in America, 1918-1920; Author Dorothy A. Pettit and Janice Bailie (Timberlane Books, 2008): The book has an excellent chapter on the biological detail of the flu virus, a historical narrative of the 1918 pandemic, and an intimate portrait of political life and social environment during the pandemic. It includes plenty of charts for statistical support.
Recommend this, Like us, Share this, retweet, #, @: